Imaging in liquid with unprecedented clarity
Researchers at the Rosalind Franklin Institute have made a significant breakthrough by imaging bacteria in liquid with unprecedented clarity. Published in the journal Small, this advancement in Liquid Phase Electron Microscopy (LPEM) allows scientists to study biological molecules in their natural state.
The team at the Franklin have been working to improve Liquid Phase Electron Microscopy methods because studying molecules in their native state without the need to chemically or cryogenically fix their samples would provide new insights to biological structures and the dynamic function. This requires imaging in liquid environments that mimic those found in vivo.
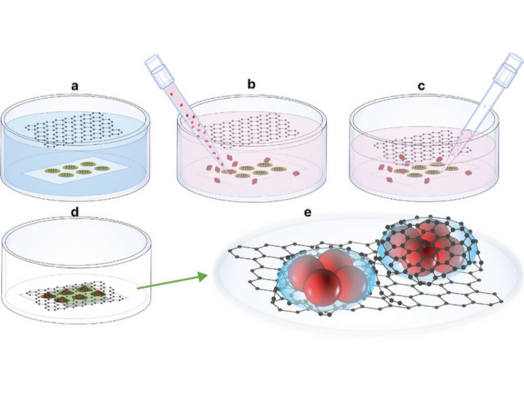
Dr Brian Caffrey, co-lead author of the paper and postdoc at the Franklin, explains what this improvement in resolution means, “People have imaged bacteria before in liquid, but usually they are only able to see the fuzzy outline of the bacteria. We can look inside the bacteria and clearly view the different layers of the bacteria’s membrane and other biomolecules.”
“Usually when samples are analysed in liquid, they’re looking through these big, thick (50 nm) blocks of silicon, which mean a lot of the imaging electrons are scattered, creating a blurry image. In comparison Adrian’s graphene, which is just one atom thick, means we’re basically looking at naked bacteria with a bit of carbon on top and that then allows us to be able to visualize the insides of these bacteria.” Dr Adrian Pedrazo-Tardajos, co-lead author of the paper and postdoc at the Franklin, said, “This graphene cell technology has not been sufficiently exploited within the life sciences, but I think there are a lot of potential applications.”
In this research, the team focused on Deinococcus radiodurans because they are thirty times more resistant to radiation than other common bacteria, such as E. coli. Their resistance to radiation damage makes them an excellent initial choice for studying sub-cellular ultrastructure using Liquid Phase Electron microscopy.
One of the ways in which D. radiodurans protects itself from radiation is by taking up manganese, and the team showed that these manganese ions were stored in phosphate-containing storage granules using Energy Dispersive X-ray Spectroscopy techniques in combination with electron imaging methods.
Dr Judy Kim, Deputy Director of Correlated Imaging and co-author of the paper, said, “Here, we were able to image on the 10s of nanometres rather than 100s of nanometres level, which is a factor of 10 improvement on where liquid phase electron microscopy was in biology, so we are very excited about this. Think about the possibilities.”
“This work represents a great step forward in what we can achieve using liquid phase imaging technology. The aim of the Franklin is to create leaps forward in technology and I believe this research is a demonstration of what was asked of us.”
The goal of this project is ultimately to be able to image cells in liquid which allows them to observe cellular dynamics and protein-protein interactions.
The team believes that the ability to view these bacterial membrane layers could also open the door to visualising dynamic processes within a cell at nanometre resolutions, leading to further insights into a myriad of intra- and extra-cellular interactions.
