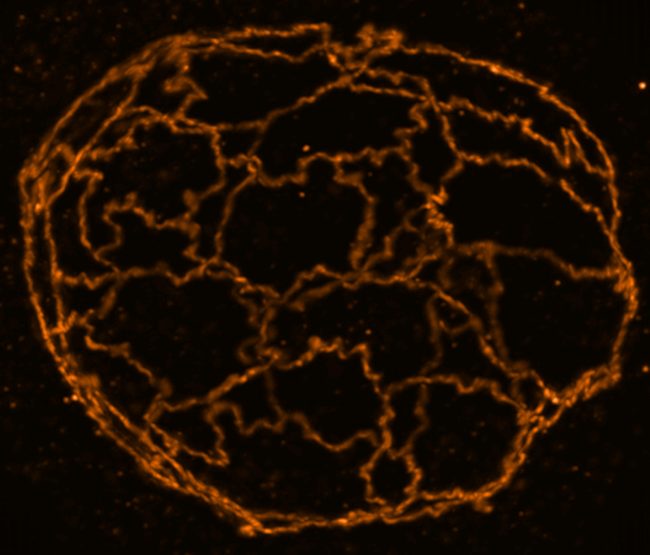
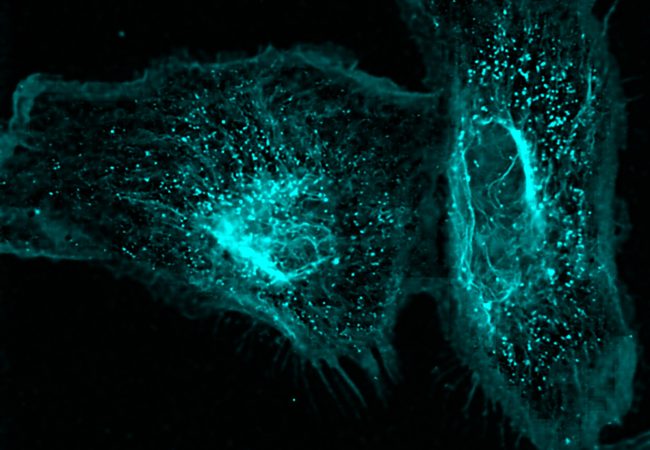
By focusing on how cells communicate with one another and how organoids and tissues form, particularly in the gut, we hope that this will lead to the opening of novel therapeutic venues for intestinal diseases.
We are interested in approaching biological questions as multiscale processes from molecules to tissues. At times, the underlying mechanisms of self-organisation of molecules and tissues might follow the same rules. We are fascinated in understanding how epithelia cells organised their components in space and time to shape a robust tissue that allows life.
Epithelial cells have developed cell-cell adhesion complexes that keep them together, forming barriers that protect us from the external world. During the cell adhesion process, cells also acquire a characteristic apical-basal polarity that drives the membrane compartmentalisation and the order of biomolecules. The loss of either cell polarity or cell adhesion leads to cell and tissue disfunction. Several diseases have been associated with loss of tissue barrier function provided via the cell adhesion complexes or the dysregulation of cell polarity, including processes such as pathogen entry, cancer progression and inflammation. However, it is still very difficult to pinpoint the direct molecular mechanism between these diseases and cell adhesion. Interestingly, despite being an important therapeutic target that affect many diseases, there are still very few small molecule drugs targeting cell junctions.
We have recently discovered the formation of biomolecular condensates on membranes following biophysical processes, known as wetting transitions, are responsible for assembling the tight junction. We also want to push the understanding of condensate formation in tissues developing different cutting-edge techniques that will allow us to follow the spatio-temporal dynamics of these processes in the human gut. We want to move beyond proteins and understand how other biomolecules such as lipids and sugars play a role on the organisation of the cell membrane during adhesion and polarity. As we face a new era on the physics of life, it is fascinating to keep exploring how physical rules, geometrical constraints and mechanics play a role on organising life beyond genes.
We seek to combine quantitative cell biology, chemical biology and biophysics to understand the spatio-temporal organisation of cell junctions in the gut and its dysregulation during inflammation and infection. Our primary technique is super-resolution STED microscopy which allows us to visualize the dynamics of nanoscale adhesion complexes inside cells. Our current set-up using adaptive optics also allows us to visualize organoids and human healthy and infected tissues in 3D, which has been a significant challenge due optical aberrations in thick samples. We hope the visualization of these processes in space and time at high resolution in 3D tissue samples will bring us a step closer to understanding the biology and link to the pathology.