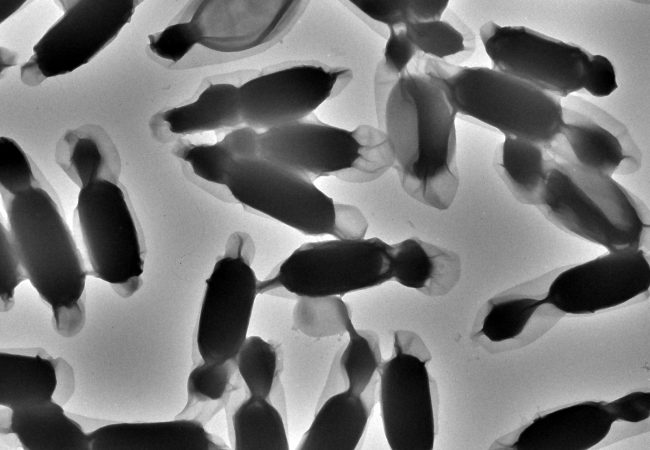
This has limited EM to dry or frozen specimens, thereby hindering the observation of these dynamic interactions. Our aim is to pioneer liquid phase electron microscopy methods that can study these structural and dynamic features in their native aqueous environments, protected from the harsh microscope vacuum. This will create new methods to better understand our biological world and generate new therapeutics informed by our observations.
Liquid Phase Electron Microscopy and Spectroscopy
Transient, dynamic assemblies of biomolecules in solution are the primary driving forces behind biology. However, studying these at high resolutions requires the use of electron microscopes (EM), which need extremely high vacuums to function.
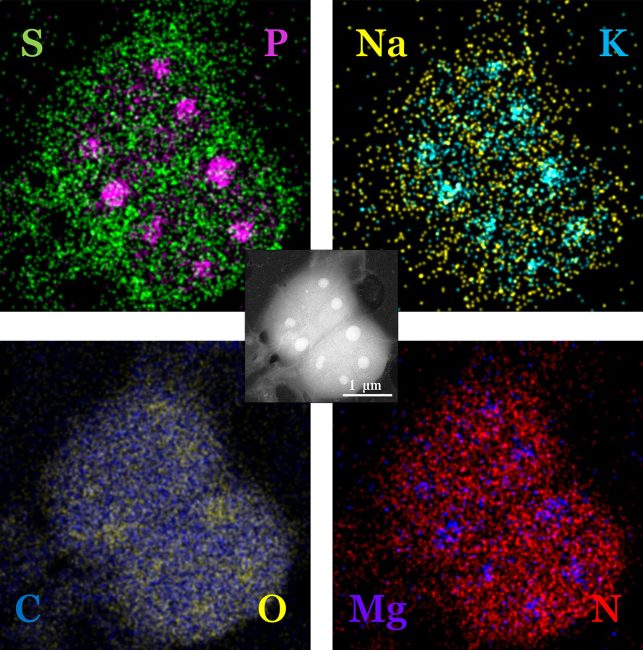
Cryo-EM’s greatest strength is the ability to achieve near-atomic level resolutions in native environments of frozen-hydrated biomolecules and cells. However, frozen samples by their very nature reduce very dynamic processes to single snapshots in time. Computational and theoretical inferences enable these snapshots to glimpse the dynamic intermolecular relationships which composes the protein structural ensemble. However, these are “best guesses” regarding the series and direction of conformational changes a given protein makes, often skewed by more stable conformations. Transitioning from single snapshots in time to movies of actual dynamics would transform how we approach protein-drug interactions, studies of protein complex formation in cells, protein aggregation, cell response/interactions and numerous other biologically and clinically relevant phenomena. The rapidly emerging field of liquid phase electron microscopy (LPEM) may be vital to unlocking the next frontier of structural biology in EM – in situ structural dynamics.
Here at the Rosalind Franklin Institute, we are testing ways to encapsulate biological specimens in new graphene and conventional silicon nitride liquid cells to protect them from the harsh vacuum of the electron microscope. This work will allow us to image life in liquid, expanding our understanding across spatial and temporal dimensions. Combined with advanced electron spectroscopic techniques we can start to build a more complete picture of the complex chemical and biological changes which occur within the cell at nanometre resolutions and millisecond timescales.

Dr Brian Caffrey
Postdoctoral Scientist

Professor Angus Kirkland
Science Director and Challenge Lead

Dr Judy Kim
Deputy Challenge Lead and Chair of the Graduate Studies Board

Dr Adrián Pedrazo Tardajos
Postdoctoral Scientist

Dr Tobias Starborg
Senior Support Scientist

Dr Mohammed Yusuf
Sample Preparation Scientist

Katie Beirns
PhD Student

Jack Bromley
PhD Student