In the initial phase of this project, we will develop a transformative cryogenic 3D biochemical microscope, harnessing the power of high-resolution electron microscopy and mass spectrometry imaging. This new tool will combine biological structural information from cryogenic Electron Microscopy (cryo-EM) and chemical information from Mass Spectrometry imaging (MSI) in order to amplify the information attained individually and ultimately to map 3D volumes at sub-cellular resolutions. The combination of these techniques will allow us to make the most out of our biological samples – this is especially important for rare or precious samples like human tissue.
Biochemical Microscopy for imaging across Molecular Scales
Developing a transformative cryogenic 3D biochemical microscope, harnessing the power of high-resolution electron microscopy and mass spectrometry imaging
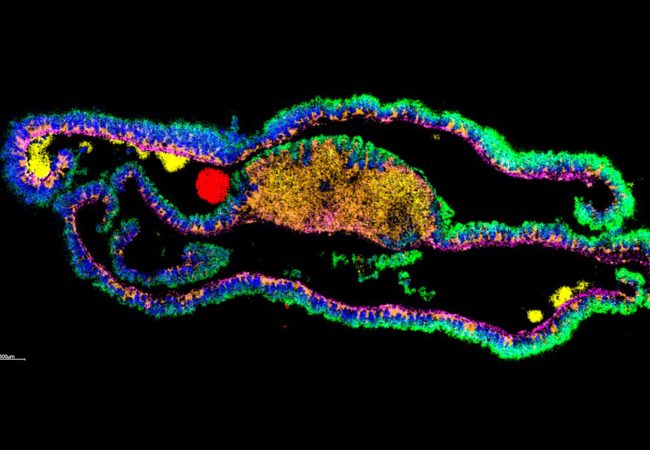
Cryo-Electron Tomography (cryo-ET) can map native vitrified cells and tissues at near atomic scale, but is limited by electron transparency to thin lamellae (~200 nm) that are prepared using focussed ion beam (FIB) milling while structural and chemical knowledge of surrounding regions is lost. Yet, the network of molecular interactions between cells is a critical component of understanding how cells communicate, divide, or are subjugated to pathogens. Mass spectrometry imaging (MSI) can be used to assess and localise molecules such as lipids, metabolites, peptides and proteins, while SEM (Scanning Electron Microscopy) can build a picture of the local morphology. Using these techniques together, we aim to exploit MSI to build up a 3D picture of the chemical and biological structure of tissue and cell environment.
Our initial focus is to exploit the 3D cryo preparative step for cryo-ET that uses plasma focussed ion beam scanning electron microscopy (pFIB-SEM) and the 3D nature of secondary ion mass spectrometry (SIMS) to enable precise label-free chemical targeting during lamella preparation. However, bridging the gap in size domains and getting structural and chemical information on the same areas of interest is a significant challenge. In this project we aim to explore how recent developments in these technologies could be used to bridge this gap and radically enhance each other through a series of proof-of-concept experiments, on cell and tissue samples, that will enable design of transformative technology in life sciences. This conceptual design would deliver a completely new type of instrument, capable of exploring biological tissues in a way previously not possible – bringing together high-resolution SEM imaging with SIMS imaging to create a single tool for 3D cryogenic biochemical microscopy at subcellular resolution.
Broadening this concept further, we plan to consider the direct analysis of protein structure -through the integration of high resolution microscopy of cryo-ET to determine protein structure and native ambient mass spectrometry which is able to chemically map proteins within their natural environment. Furthermore, we plan to harness native ambient mass spectrometry to extract, separate, and capture protein complexes from tissue for subsequent super resolution mass spectrometry.
These developments will transform interpretations of complex cellular images by providing chemical information that will shine a light on cell functions, as well as structure. Fundamental processes, such as neuronal connectivity, dysregulation leading to cancer, and infection are chemically driven, but this can only be understood in the context of the molecular structures that drive cellular changes affecting function.

Dr Gwyndaf Evans
Head of Technology

Dr Maud Dumoux
Deputy Challenge Lead and Technology Lead for Cryo Imaging

Dr Felicia Green
Deputy Challenge Lead and Senior Scientist

Professor Helen Cooper
Science Director and Challenge Lead

Dr Michael Grange
Emerging Interest Area Lead and Tomography Group Leader

Professor Angus Kirkland
Science Director and Challenge Lead

Dr Jiaqi Luo
Postdoctoral Research Associate

Dr Jianguo Zhang
Senior Application Expert for in situ Structural Biology

Andy Stallwood
Mechanical Design Engineer

Dr Matija Lagator
Postdoctoral Research Associate in Secondary Ion Mass Spectrometry
Ionoptika Ltd
The instrument developed in collaboration with UK based Ionoptika enables a dramatic improvement in mass spectrometry imaging (MSI). The new instrument is capable of imaging the whole surface simultaneously, allowing more accurate imaging at faster speeds than was previously possible.
Thermo Fisher Scientific
Researchers at the Franklin have been working closely with Thermo Fisher Scientific through our Wellcome funded ‘Electrifying Life Sciences’ programme to develop the next generation of electron microscopes for imaging the smallest structures of cells in intact tissues, bring these tools to market and establish new methods and standards for the community to enable their wide use.