- Multiomic chemical imaging
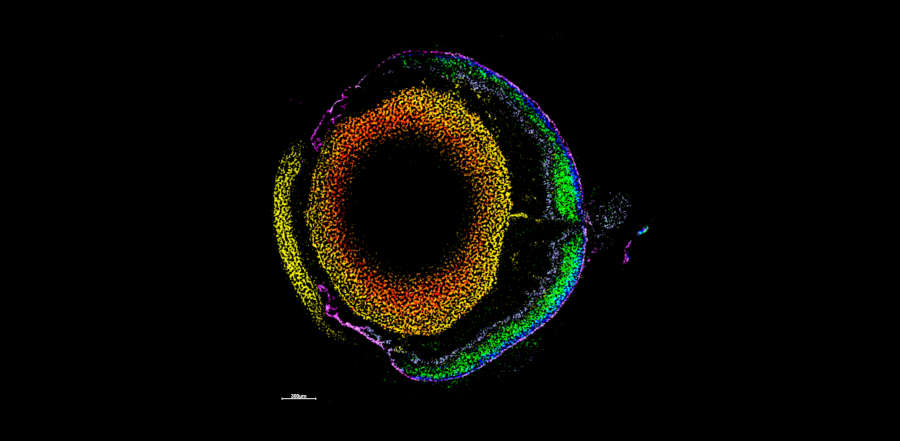
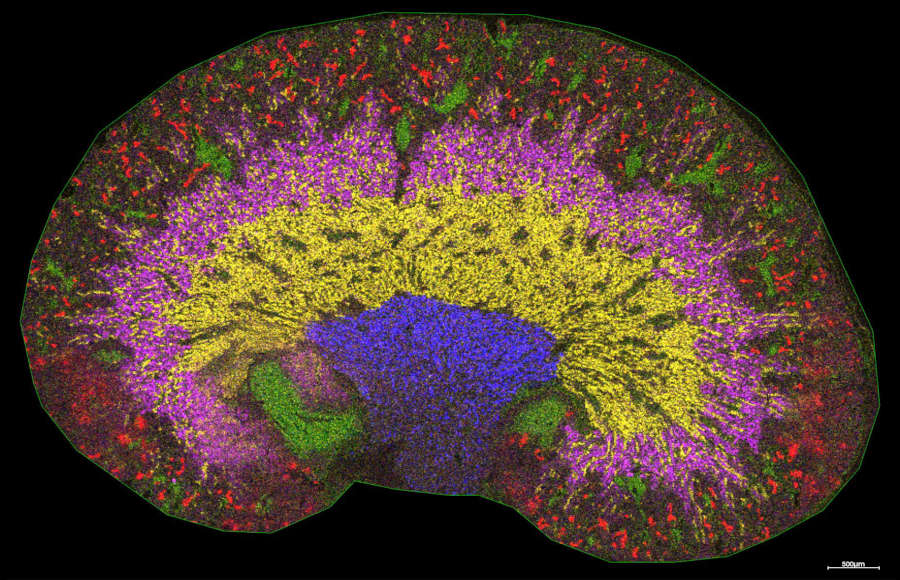
- Dissecting the glyco-environment of tissues during different disease states
- Investigations into the gas phase structures of ions
The TIMS-TOF MS enables rapid, confident identification and analysis of biomolecules such as peptides, lipids, metabolites and glycans.
The TIMS-TOF is a dual ion source (electrospray ionisation, ESI, and matrix assisted laser desorption ionisation, MALDI) mass spectrometer. Access to both sources enables a myriad of different workflows to be undertaken, ranging from simple direct infusion experiments, through complex liquid chromatography analyses to deep imaging investigations into the chemical state(s) of tissue. A high sensitivity, rapid (10 kHz) time of flight (TOF) mass analyser is used, which enables the analysis of the chemical contents of single cells at high speed.
The main advantage of a TIMS-TOF is access to the trapped ion mobility spectrometer (TIMS) device. Ion mobility spectrometry separates by the collisional cross section or size of a molecule to charge ratio, instead of the usual mass to charge ratio (m/z) of ions that is investigated in mass spectrometry (MS). Mobility separation is orthogonal to mass separation, enabling the discrimination of structural- and stereo- isomers whilst also increasing the signal/noise ratio of MS measurements by separating complex ion populations into smaller, discrete mobility populations before mass analysis.
TIMS offers one of the highest, commercially available, ion mobility resolutions (>400, compared to a standard drift tube which operates mostly at ~50). Access to such resolution enables separation and analysis of incredibly similar molecules: molecules such as carbohydrates, which can differ by as little as the direction of a single hydroxyl and even different conformations of the same molecule, e.g. a helical or linear conformation of a peptide. Traditionally, some kind of off-line or in-line separation (such as liquid chromatography; LC) is required to separate such similarities. These methods can take anywhere from a few minutes to hours or days to complete. TIMS offers comparable separation but on the millisecond time scale. The “trapped” nature of the device also enables mobility separated ions to be stored for short periods of time, allowing manipulation of one population of ions whilst the others are kept, this increases duty cycle up to 100%, improving throughput whilst retaining high sensitivity.
At the Franklin, the TIMS-TOF is primarily used for MS imaging (MSI) method development, where we are coupling rapid TIMS measurements with traditional MSI methodologies to uncover an “LC-like” separation on a pixel-by-pixel basis, thus revealing further the chemical complexity and depth of the tissue and enabling the analysis of classically under-studied molecules, such as carbohydrates. Studies into the native states, chemical composition and location of carbohydrates in situ are undertaken, as well as analysis of the metabolite and lipid populations, enabling a true multi-omic analysis of samples. TIMS is also used to study the structure and fragmentation pathways of pharmaceutical molecules, improving our understanding into their activities.