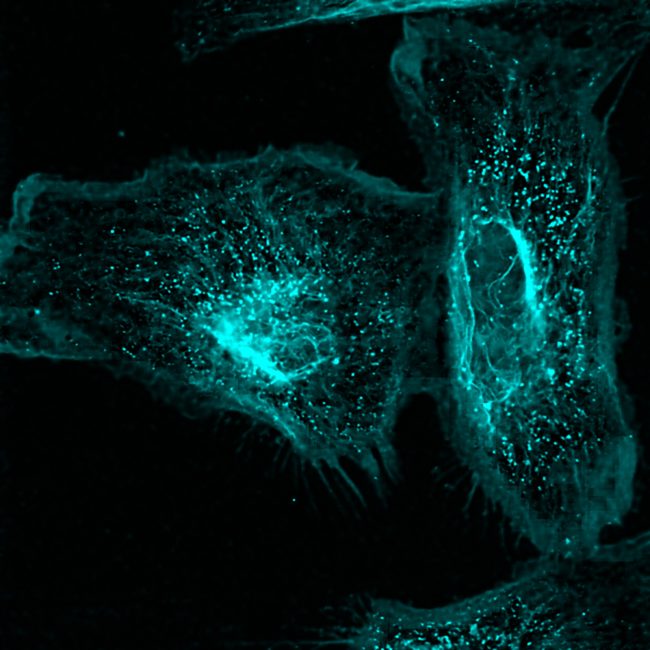
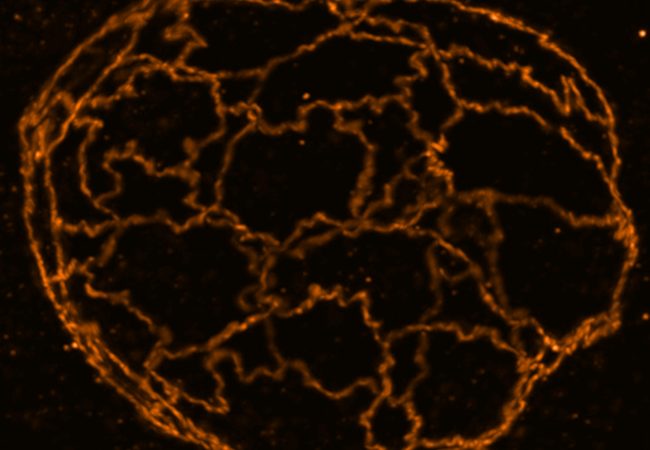
We can achieve this using a custom-built super resolution STED microscope that achieves nanoscale resolution below 20 nm to observe, for example, cell adhesion networks.
The Stimulated Emission Depletion (STED) microscope enables a super-resolution microscopy technique that enhances the capabilities of conventional confocal microscopes by incorporating a second depletion laser. This laser is shaped like a doughnut, with the outer ring actively depleting fluorescence, while the centre remains unaffected, allowing fluorescent light emitted from the focal point to be detected. This innovative addition increases resolution by a factor of ten — from 200 nanometres to approximately 20 nanometres — enabling the visualization of fine intracellular structures, such as detailed membrane, rather than just the overall shape of features in cells.
STED microscopy also enables multiplexing, allowing the visualization of multiple biomolecules—such as proteins, lipids, or glycans—labelled with fluorescent tags in a single sample. This capability facilitates detailed imaging in different colours, offering insights into multiple cellular structures and processes. By providing the option of real-time live imaging or studying fixed samples, STED helps unravel the structure and dynamics of various biological processes with high spatial resolution in both living and static cells.
Our STED microscope employs adaptive optics with deformable mirrors to correct aberrations commonly encountered when imaging complex samples. These enable the study of organoids in 3D, even with very thick samples that were previously challenging to analyse. Using super-resolution microscopy, we focus in particular on epithelial tissues, particularly the gut, to visualize the intricate process of complex cell formation within the context of health and disease.