Aberration-corrected transmission electron microscope
Ruska is an aberration-corrected transmission electron microscope (TEM) used to explore novel methods to study radiation sensitive specimens such as biological materials that have been cryogenically preserved or encapsulated in liquid for dynamic observations.

Alternative electron optical setups and scan patterns in scanning-TEM (STEM) mode are used to control exposure and experimental design for the advancement of imaging techniques such as electron ptychography, compressive sensing, or scanning electron diffraction.
Ruska is a double aberration-corrected (S)TEM with a JEOL GrandARM2, FHP2 base. The triple anticontamination device design allows for long cryogenic experiments with 2 nm/hour contamination with side-entry cryo holders. This design allows for flexibility of experiments with in situ liquid flow holders, energy dispersive x-ray spectroscopy (EDX) with a large solid angle and high-tilt tomography holders.
The edition of counting detectors enables 4D-STEM types of data collection necessary for low dose electron ptychography, scanning electron diffraction, and tilt-corrected bright-field imaging. By implementing bespoke scripting in Python, Julia and PyJEM, automated collection and reconstruction of exit waves can be viewed within minutes, while sitting at the microscope. Communication and triggering between APIs and the electron dose modulation (EDM) IDES system has been established as essential to work on radiation-sensitive biological and protein specimens. The electrostatic fluence (dose) control for cryogenic work has been introduced by this team for the first time, as it was found to be essential to perform alignment at higher fluence, then move to lower fluence for data collection, without altering imaging aberrations.
Dose-sensitive work is also addressed using a more traditional STEM optical mode, but with alternative scan patterns and dose painting of individual pixels. Using arbitrary scan generators, Ruska team members have been able to reduce specimen damage using the same total fluence as traditional raster scans.
In conjunction with our in-house expertise in graphene liquid cells, Ruska is used to create high-resolution images of cells and proteins in their native liquid buffer environment. By combining with scanning methods, EDX elemental information can be simultaneously collected to elucidate the difference between components such as stress granules appear the same in imaging modes.
- Operating energies: 40, 60, 80, 200 and 300 keV
- Aberration correction: JEOL aberration correction of probe and image with three dodecapoles for efficient spherical and 6-fold astigmatism correction.
- Operating temperatures: 80 K (cryo) or 300K (room temperature)
- Detectors: Medipix3 Quad, Direct Electron Celeritas, JEOL SAAF Octa, JEOL HAADF-LAADF-BF, Gatan K2, Gatan Orius
- Electron fluence control: IDES Synchtrony + EDM, IDES Relativity, Protochips Axon Dose
- In Situ Environmental control: Protochips Poseidon, DENS Stream, Graphene-based systems
- Scan generating systems: JEOL, Quantum Detectors, Direct Electron
- Operating modes/capabilities: TEM, STEM, Nanobeam scanning diffraction, Electron ptychography in the far-field and near-field, Fourier ptychography, iDPC, Compressive sensing, TCBF

Professor Angus Kirkland
Science Director and Challenge Lead

Dr Judy Kim
Deputy Challenge Lead and Chair of the Graduate Studies Board

Dr Brian Caffrey
Postdoctoral Scientist

Dr Adrián Pedrazo Tardajos
Postdoctoral Scientist

Dr Marcus Gallagher-Jones
Senior Electron Microscopy Structural Biologist

Dr Abner Velazco
Staff Scientist

Dr Jingjing Zhao
Postdoctoral Scientist

Dr Emanuela Liberti
Staff Scientist

Dr Ali Mostaed
Staff Scientist

Dr Tobias Starborg
Senior Support Scientist

Dr Jonathan Barnard
Senior Staff Scientist

IDES
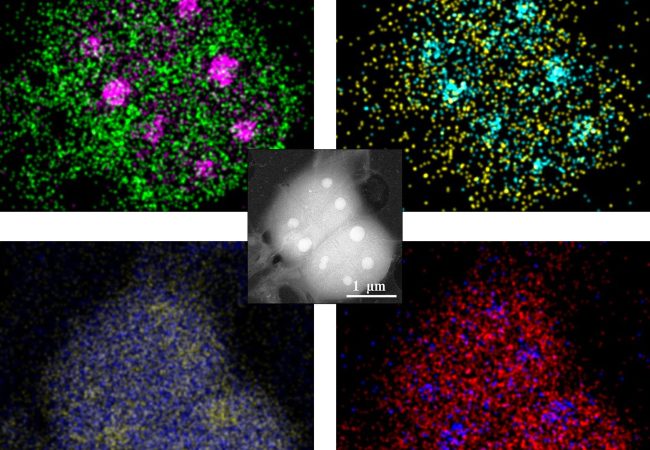
Liquid Phase Electron Microscopy and Spectroscopy
Transient, dynamic assemblies of biomolecules in solution are the primary driving forces behind biology. However, studying these at high resolutions requires the use of electron microscopes (EM), which need extremely high vacuums to function.
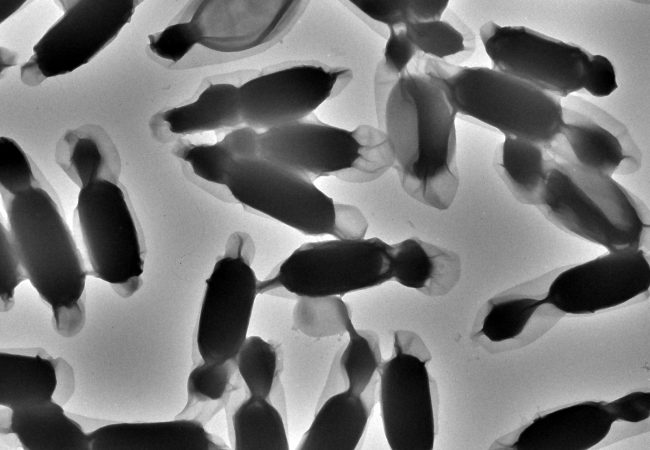
Cryo-ptycho-tomography
Developing a novel technique using cryo-electron ptychography to perform tomographic characterisation of biological processes at cellular scales, enabling detailed study of rare and complex structures in their native environments.

Electron Diffraction
MicroED is an emerging technology that exploits the strong interaction of electrons to reveal the structures of molecules from vanishingly small crystals.
