Peering into subcellular architecture at unprecedented resolution
By integrating hardware and software developments, researchers at The Rosalind Franklin Institute are paving the way for imaging the structure of molecules in cells and tissues at near-atomic resolution.

The aim of the Wellcome-funded project ‘Electrifying Life Sciences’ is to visualise molecular structures in the context in which they function. Researchers at The Rosalind Franklin Institute are redefining the capabilities of cryo-electron microscopy (cryo-EM), an established method for determining the three-dimensional structure of isolated proteins and protein complexes.
Michael Grange, a structural neurobiologist, and colleagues at the Institute, are working on protocols to facilitate the analysis of macromolecular structures in cells and tissues. In April 2023, using a new approach involving cryo-plasma Focused Ion Beam (FIB) and Scanning Electron Microscope (SEM) prior to imaging with a Transmission Electron Microscope (TEM), they reported the structure of the human ribosome within plungefrozen HeLa cells at 4.9 Å resolution1 .
By combining different imaging modalities, Dr Grange’s ultimate goal is to understand disease at the molecular level. “Current histopathology methods are quite coarse and qualitative,” he says. “Identifying the first signs of pathology at the molecular level would enable early intervention and could drastically improve disease outcomes.” What’s more, he wants to make the workflow more accessible, so a wider range of researchers can study proteins working inside cells at atomic resolution.
High-resolution 3D mapping of cells
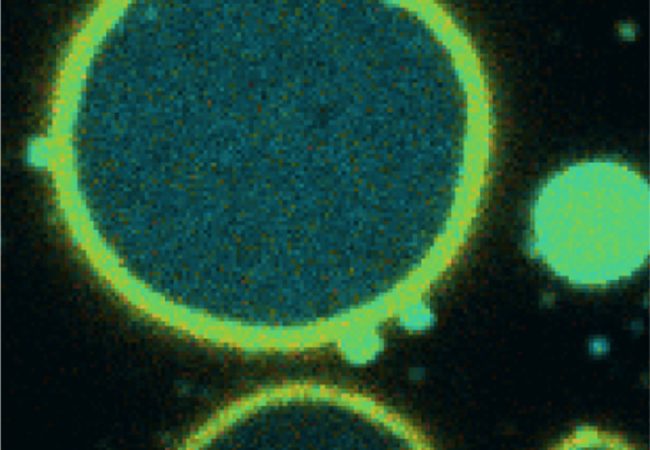
Cryo-EM involves imaging biological samples that have been plunged into liquid ethane. This results in rapid freezing of the water into vitreous ice containing the suspended samples and avoids the use of chemical fixatives and stains, which can cause unwanted structural changes and introduce artefacts. Although vitrification preserves samples in a hydrated and nearnative state, it also makes handling and imaging them more challenging.
Because most cellular samples are too thick to image with a transmission electron microscope (TEM), they need to be thinned or milled. Thin sections of sample (known as lamella) can be produced on a scanning electron microscope fitted with a focused ion beam and a cryo stage (cryo-FIB/SEM). To date, this has been a very time-consuming process that requires highly trained users.
Working with microscopists and data analysts at The Rosalind Franklin Institute, Dr Grange is pioneering methods to successfully image thicker samples at speed.
“We have been the first to demonstrate the use of plasma for FIB milling, which has potential to make the milling process less damaging and much faster,” he says. FIB systems typically rely on gallium ion beams which have lower milling speeds and are limited to lower currents. However, with plasma FIB (pFIB) generated from a range of gases (oxygen, nitrogen, argon and xenon) milling can proceed quicker and enable very thick specimens to be prepared in a matter of hours rather than days.
Additionally, with cryo-pFIB/SEM they have imaged a range of biological specimens including bacteria, cells and mammalian tissues and obtained subcellular information that helps locate regions of interest for higher resolution imaging by TEM 2 . The serial pFIB/SEM images can be correlated with fluorescence and TEM images, highlighting the promise of the workflow for multi-scale analysis.
Optimising EM-data analysis
Reconstructing 3D structures from 2D electron microscopy data requires specialised software and large computational resources. Michele Darrow, Head of Data Strategy for Cryo Electron Imaging, and her team are developing machine learning and informatics-based tools to process the data collected from cryo-pFIB/SEM and TEM.
“We’ve been working on an automated alignment package to process serially acquired cryo-pFIB/SEM images and on ways to computationally detect and remove artefacts,” she explains. Such artefacts include dark spots with asymmetric streaks, known as ‘charging’, which can occur when the electron beam interacts with lipids in the sample.
Dr Darrow and colleagues are also developing machine learning algorithms to aid image segmentation and quickly assess resolution across an image. “We can train models to specifically classify voxels into discrete categories, which is helpful to identify organelles and their relative location within a cell,” she says.
As a champion of open-source software, The Rosalind Franklin makes all software tools available on GitHub. Furthermore, to make the new tools easier to use by people who are not computer scientists, they are integrated into commonly used software packages such as napari, a Python-based tool for viewing and analysing multi-dimensional images.
Pushing boundaries
While Dr Darrow is working to further test the software for different imaging modalities and purposes, such as analysing larger sample volumes, Dr Grange is collaborating with colleagues across the UK and Europe to build high-resolution 3D pictures of brain tissue. Interactions between cell components could provide key insights into neurodegenerative diseases.
“Rather than study isolated amyloid fibrils from patients with Alzheimer’s disease, for example, we want to look at them in the tissue environment and see how they impact other cell structures,” Dr Grange says. “The tools we build here will enable us not just to investigate the molecular organisation of brain diseases, but a whole range of tissues, from cardiomyopathies to liver or kidney dysfunction.”
1. Berger, C., Dumoux, M., Glen, T. et al. Plasma FIB milling for the determination of structures in situ. Nat Commun 14, 629 (2023).
2. Dumoux, M., Glen, T., Smith, J.L.R. et al. Cryo-plasma FIB/SEM volume imaging of biological specimens. eLife 12, e83623 (2023).