Optimised sample preparation fuels the ‘resolution revolution’
Researchers at The Rosalind Franklin Institute are working with SPT Labtech and Diamond Light Source on chameleon®, an automated sample preparation system that improves the efficiency of the cryo-electron microscopy workflow.
As one of the Franklin’s founding members, we have worked closely with Diamond Light Source from our inception. A commissioning call through the Diamond eBIC facility enabled first time community access to key components of our cryo-electron tomography pipeline.
As cryo-electron microscopy (cryo-EM) becomes more sensitive and more widely available, biologists are increasingly drawn to this technique to solve the 3D structure of proteins. These structures are fundamental for understanding protein function, their potential role in disease and, crucially, for designing drugs that target them.
Cryo-EM involves flash-freezing solutions of proteins or other biomolecules before showering them with electrons to reconstruct their three-dimensional shape in atomic detail. Unlike X-ray crystallography, cryo-EM doesn’t require protein crystals, which can be challenging to grow and lock proteins in a single conformation. With cryo-EM, proteins are free to move around until the moment of flash-freezing so researchers can capture different conformational states that can offer a deeper understanding of their mechanism of action.
‘Thanks to improvements in hardware and software, we’ve seen a big jump in the quality of the structures solved by cryo-EM,’ says Paul Thaw, Product Manager for Integrated Structural Biology at SPT Labtech. ‘Scientists are realising that they can quickly create high-resolution models of molecules with multiple components in varying biologically relevant states, which has traditionally been very difficult to do with X-ray crystallography, either because of their complexity or inherent instability.’
What is often referred to as the ‘resolution revolution’ in cryo-EM has resulted in a steady growth in the number of structures solved by this technique and submitted to the Electron Microscopy Data Bank. In the next few years, the number of biological structures determined by cryo-EM is likely to surpass those determined by X-ray crystallography 1 .
Tackling the main workflow bottleneck
Cryo-EM relies on a sample preparation method known as vitrification. This involves immobilising the biological specimen on a support, a so-called grid, by rapidly freezing it into a glass-like or vitreous state, avoiding the formation of ice crystals that can compromise the structure of the specimen.
As Dr Miriam Weckener, Postdoctoral Research Scientist in Structural Biology at the Franklin, explains, sample preparation for cryo-EM is by far the most timeconsuming and difficult stage of a project. Samples need to be carefully applied to a grid, blotted and quickly plunged into liquid ethane.
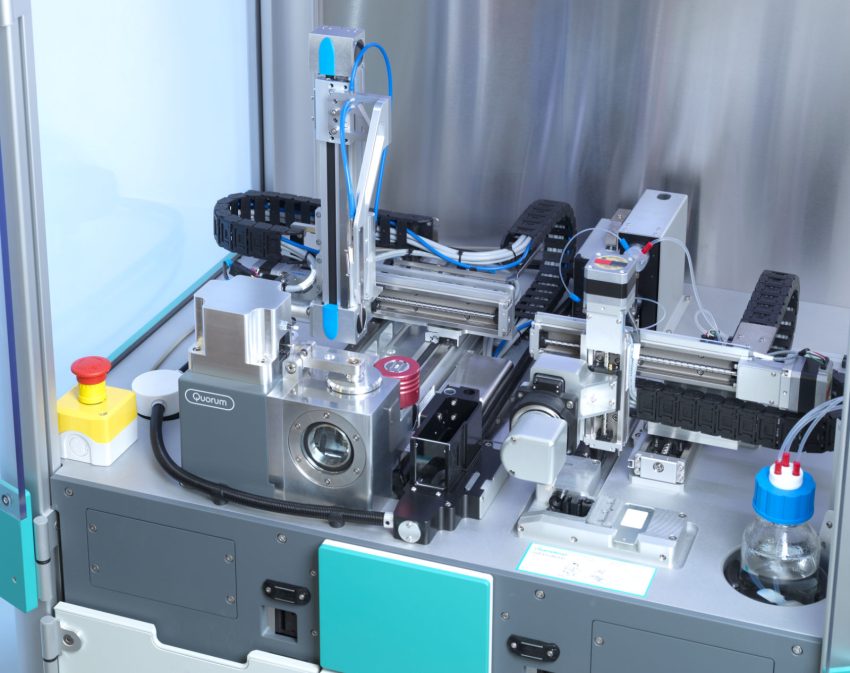
chameleon® uses inkjet technology to spray tiny amounts of sample (minimum dispense volume 6nL) onto unique self-blotting nanowire grids. ‘The whole system is automated, minimising grid damage and sample waste caused by manual handling,’ she says.
Moreover, chameleon®’s high-speed cameras allow fine control over sample layer thickness. ‘When using older semi-automated methods, you have no idea of the ice quality of the sample on the grid until you put it in the microscope,’ Dr Thaw explains. chameleon® allows researchers to discard samples that are too thick or too thin so valuable microscope time is not wasted screening unsuitable grids.
Other parameters that can be fine-tuned with chameleon® enable a very short time (54 milliseconds) for sample application and freezing, which can prevent the sample from adopting a preferred orientation or dissociating (falling apart). ‘Preferred particle orientation is a common problem for cryo-EM; if we only see a protein in one orientation, we can’t accurately reconstruct its 3D structure,’ Dr Weckener explains.
By using chameleon® to optimise sample-specific preparation and control grid quality, researchers can generate consistent results faster and with smaller amounts of sample.
Putting the system to the test
SPT Labtech have been working with researchers at the Institute on chameleon® since 2019, first on an early prototype and since November 2020 on an upgraded production-level model. ‘It has been fantastic to work with scientists at The Rosalind Franklin Institute; like us, they are not afraid to tackle the more challenging aspects of the cryo-EM sample preparation,’ says Dr Thaw. ‘They tell us what works and what aspects need improving.’
Dr Weckener has been optimising the system for different types of samples, including nanobodies in complex with the spike protein of SARS-CoV-2 that mediates viral entry into cells2 . This work shed light on the structural basis of SARS-CoV-2 neutralization, which is aiding the development of new COVID-19 therapies.
By reaching out to Diamond’s EM user community, Weckener has been able to test samples that have proved difficult to prepare for cryo-EM on chameleon®. ‘We’ve been offering 2-day sessions during which we make a series of grids using different parameters to try to solve all sorts of issues encountered using other systems,’ she says. Some of these sessions have resulted in long-term collaborations, including one with a Korean group that is helping to optimise the grids themselves.
Dr Weckener has found the partnership with SPT Labtech very rewarding. ‘Developing new instrumentation is an iterative process; it is very gratifying that SPT Labtech take our feedback on board and that we are able to offer a support service to the community.’
Speaking about the future, Dr Thaw says that SPT Labtech plans to further develop chameleon® and a broader range of grids, as some samples may be better suited to grids made of different materials. In addition, he is keen to collate all the data produced in the process and apply emerging computational methods to build a knowledge base that will enable the cryo-EM community to adjust the sample preparation protocol according to the type of sample they are studying.
‘Ultimately, our goal with chameleon® is to democratise the use of cryo-EM by bringing vastly improved and more efficient sample preparation closer to the bench biochemistry,’ he concludes.
“It has been fantastic to work with scientists at The Rosalind Franklin Institute; like us, they are not afraid to tackle the more challenging aspects of the cryo-EM sample preparation”.
References
1. Nature 578, 201 (2020). https://doi.org/10.1038/ d41586-020-00341-9
2. Nat Struct Mol Biol 27, 846–854 (2020). https://doi. org/10.1038/s41594-020-0469-6